dye-sensitized solar cells(DSSCs) convert solar energy into electrical energy.
DSSCs imitate how plants harness solar energy.
In DSSCs electrons originate from a dye when it absorbs light, the dye contains a conjugated system that has alternating single and double bonds that absorbs light in the visible spectrum.
Before diving into the operation of the dye-sensitized solar cells we’d first like to talk a bit about the structure.
Setup of a Dye Solar Cell:
It consists of :
- An Anode
- A cathode.
- A semiconductor: titanium dioxide (TIO2).
- Dye-Molecule: ruthenium polypyridine dye attached to the semi-conductor.
THE WORKING OF THE DSSCs:
How does the dye-sensitized solar cell actually work?
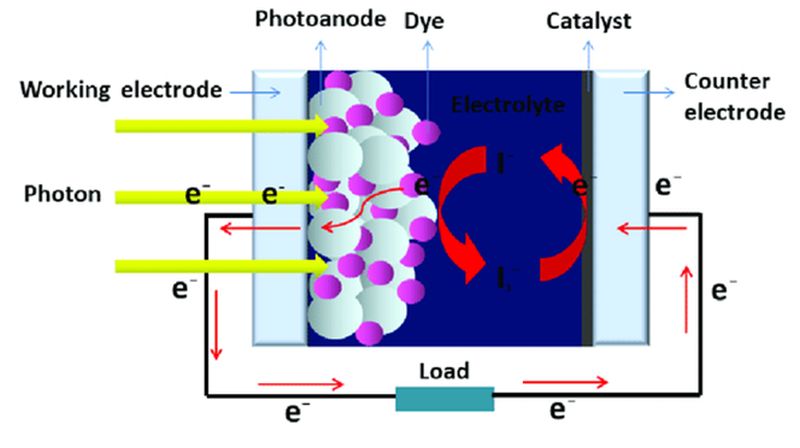
Well we’re going to dive into the mechanics of this solar cell technology and how they end up being solar cells so DSSCs can be used for different applications, first as shown above in
the structure of DSSCs you see that you have two glass slides and then a bunch of functional materials in between.
So you see an image of what it can look like where you have two glass slides, a light source, and in between, you have all these sandwich materials.
1. A Light Source
Okay, starting off we’re always going to need a light source such as the sun but this can also use indoor lighting such as bulbs, and light produces photons the packets of light energy and that’s where everything starts.
The idea behind incident photons from the light source is that they excite whatever molecule they run into providing electrons.
2. The Dye Molecule
The dye can be also called photosensitizers, is mostly ruthenium polypyridine dye or various organic metal-free compounds.
So The incident photons run into the dye molecule that can absorb the energy of light, exciting the molecule.
what does this mean? This means that is that electrons are now free to move around
and no longer completely bound to the state where it was before, so the excited dye molecule injects electrons into the titanium dioxide layer which acts as a semi-conductor.
The injected electrons leave holes behind in the dye molecule.
3. A SemiConductor
Titanium dioxide (TIO2) is used as a semiconductor that exhibits a high surface area because of its high porosity.
So we have an electron and the hole to be able to utilize this energy now stored in the excited electron we need to close the loop to be able to get an electric current flowing we need to close the loop so the electron goes from the dye molecule around into whatever load (such as a bulb or whatever) we’re going to use.
How is that done? well first off as we mentioned before the dye molecules are attached to our
Semiconductor which is titanium dioxide.
The semiconductor layer isn’t just a flat layer it’s a nanoparticle network
why would you use a nanoparticle Network? well, the idea behind using nanoparticles is to increase the surface area efficiency because they have a very large surface area.
So even though they’re so small and make a quite thin layer you will have a huge surface area and this is a really important feature because you want as many dye molecules as possible to sit on this surface.
In addition to that allows more light over a wider range of the visible spectrum to be absorbed
this allows the DSSC to absorb more light under cloudy conditions than silicon-based
photovoltaic cells.
4. The Anode
The slide of glass shown on the left side layered with a thin transparent conductive oxide (TCO) is the anode.
So as we showed as the dye molecule is excited electrons are injected into the semiconductor layer and these electrons will eventually end up on the working electrode -(the anode)- the
An example of one of the most common transparent conducting oxides is indium tin oxide, what transparent conductive oxide does is providing a route for the electrons so the electrons can now go with less resistance through this layer until it reaches a contact that we make like a copper wire or something where the electrons can flow easily.
Then we connect the wire to some kind of load, maybe this is a light bulb or a pump or a battery or whatever you like it to be and then the electrons flow to the other polypyridine ide of the solar cell.
5. The Cathode
The slide of glass shown on the right side of the figure is layered with a thin Pt film which catalyzes the cathode.
An iodide and tri-iodide are used as an electrolyte.
The electrons flow through the external circuit to the Platinum cathode, they then flow into the
iodide electrolyte.
the electrolyte transports the electrons back to the dye molecules in the DSSC, the dye molecule is oxidized it loses an electron.
the oxidized dye receives an electron from an iodide ion which reduces the dye back to its original form.
In this process the iodide ions undergo oxidation
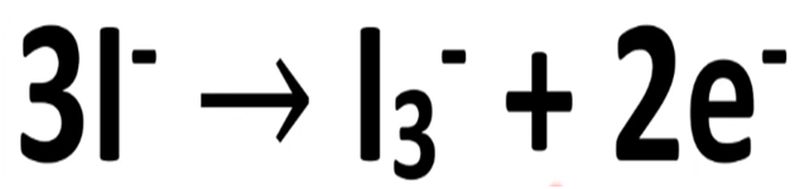 in this equation, we have the oxidation of iodide ions to form the tri-iodide ion and two electrons. These electrons reduce the oxidized dye back to its original form.
in this equation, we have the oxidation of iodide ions to form the tri-iodide ion and two electrons. These electrons reduce the oxidized dye back to its original form.
Electrons that return to the DSSC from the external circuit reduces the
tri-iodide ion back to iodide ions.
 In this equation, we can see the reduction of the tri-iodide ion to form iodide ions the iodide ions can then be oxidized to form tri-iodide ions and electrons.
In this equation, we can see the reduction of the tri-iodide ion to form iodide ions the iodide ions can then be oxidized to form tri-iodide ions and electrons.
Last Words
As we promised, we provided you with a full definitive guide on the mechanics of the DSSC technology and how they end up being solar cells so DSSCs can be used for different applications.
In the end, we hope you enjoyed this article, and stay tuned for more awesome solar tips and tricks from Solar Energy Hackers.
Do you find this guide helpful? Do you have any questions or ideas to make this guide even better?
Please, let us know your thoughts in the comment section below.
Article Submitted By Community Writer