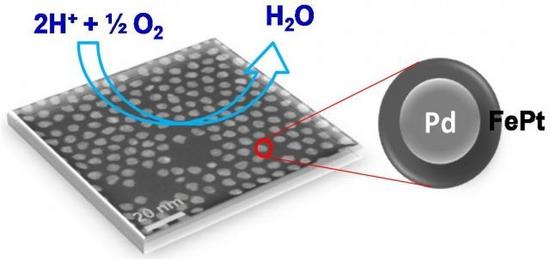
Pricey platinum does not do well as catalyst when exposed to fuel-cell reactions for long. So, to avoid the frequent use of pure-platinum catalysts, a team of chemists at Brown University has created a unique core and shell nanoparticle that continues to operate through the oxygen reduction reaction at the cathode end of a fuel cell. The nanoparticle is a five-nanometer palladium (Pd) core enveloped by a shell that consists of iron and platinum (FePt).
While retaining its original shape, the shell requires the smallest amount of platinum for an efficient reaction. The team decomposed iron pentacarbonyl [Fe(CO)5] to reduce it to platinum acetylacetonate [Pt(acac)2]. The shell used only 30 percent platinum. Laboratory tests reveal that the palladium/iron-platinum nanoparticles generated 12 times more current than traditional catalysts, consistently over 10,000 cycles. The U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy funded the research that was conducted by Brown graduate student and co-author Vismadeb Mazumder and researchers from Oak Ridge National Laboratory in Tennessee.

Via: InSciences

